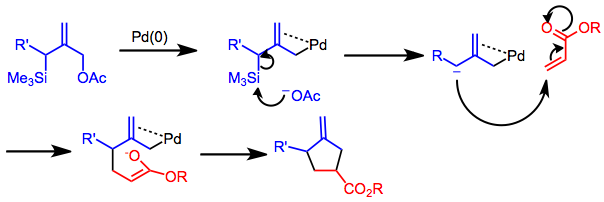
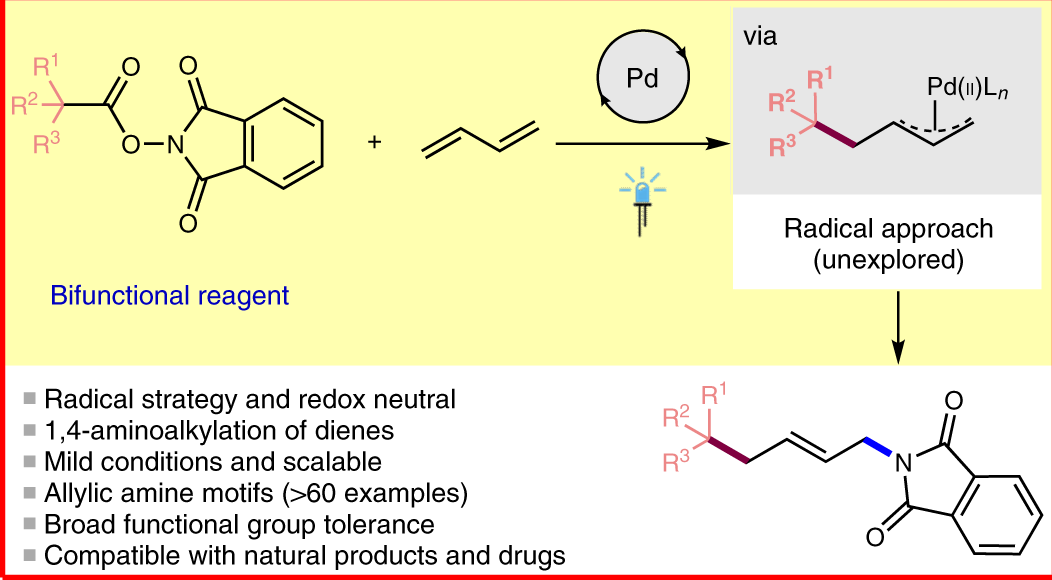
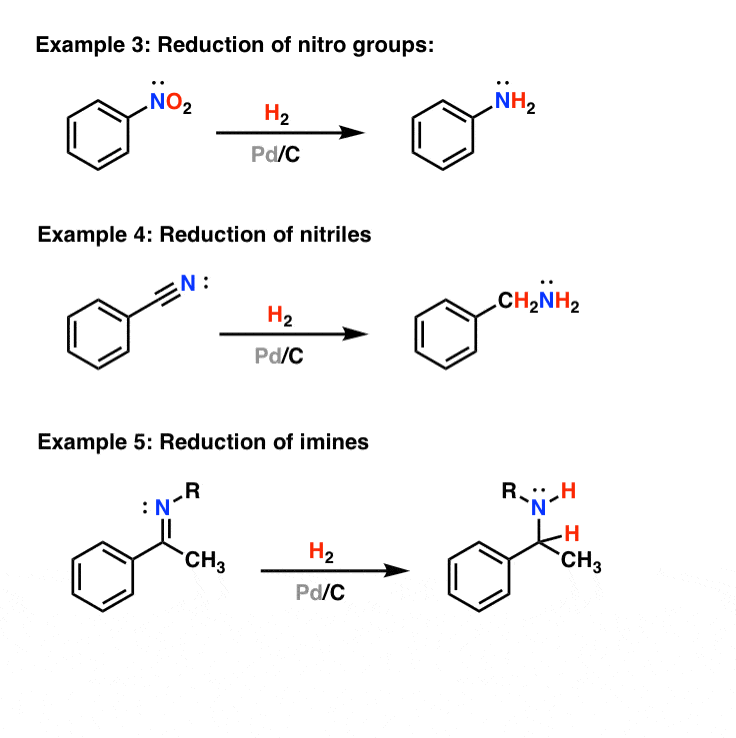
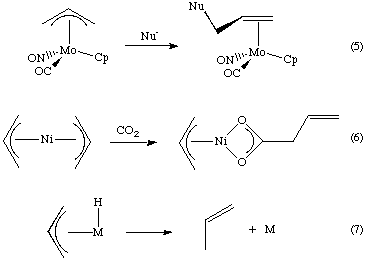
Palladium-catalyzed reaction of γ-silylated allyl acetates proceeding through 1,2-shift of a substituent on silicon - ScienceDirect

Base-catalyzed conversion of π-alkene–palladium chloride complexes into π- allyl complexes - Chemical Communications (London) (RSC Publishing)

O2-promoted allylic acetoxylation of alkenes: Assessment of “push” versus “pull” mechanisms and comparison between O2 and benzoquinone - ScienceDirect
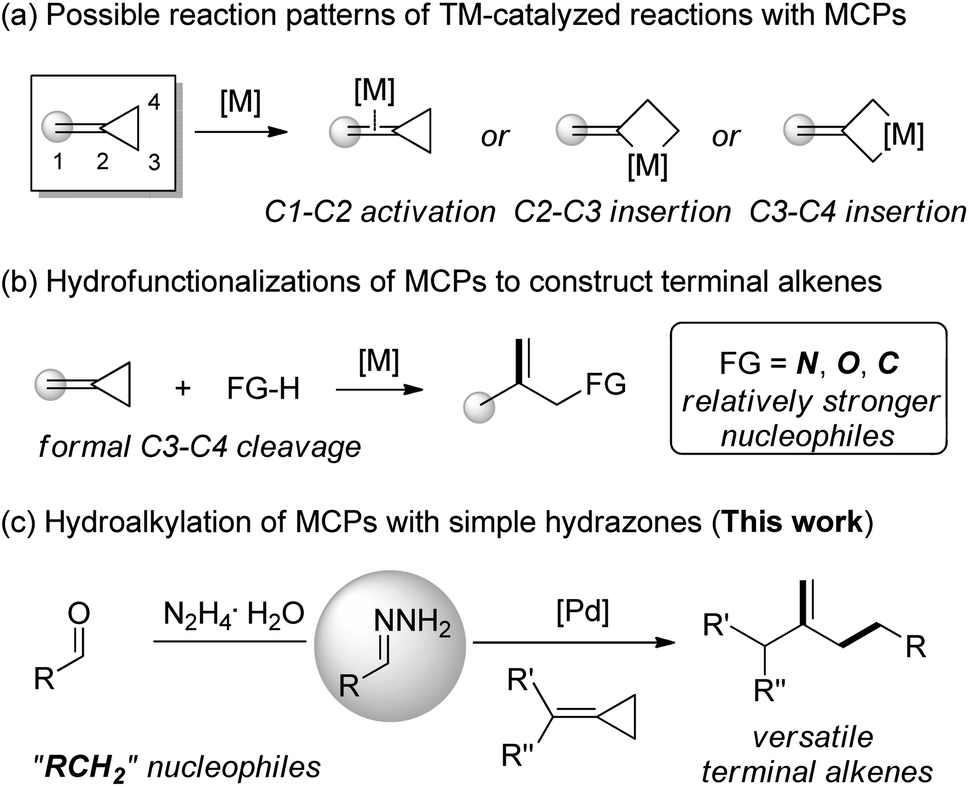
Palladium-catalyzed hydroalkylation of methylenecyclopropanes with simple hydrazones - Chemical Science (RSC Publishing) DOI:10.1039/D0SC01221A
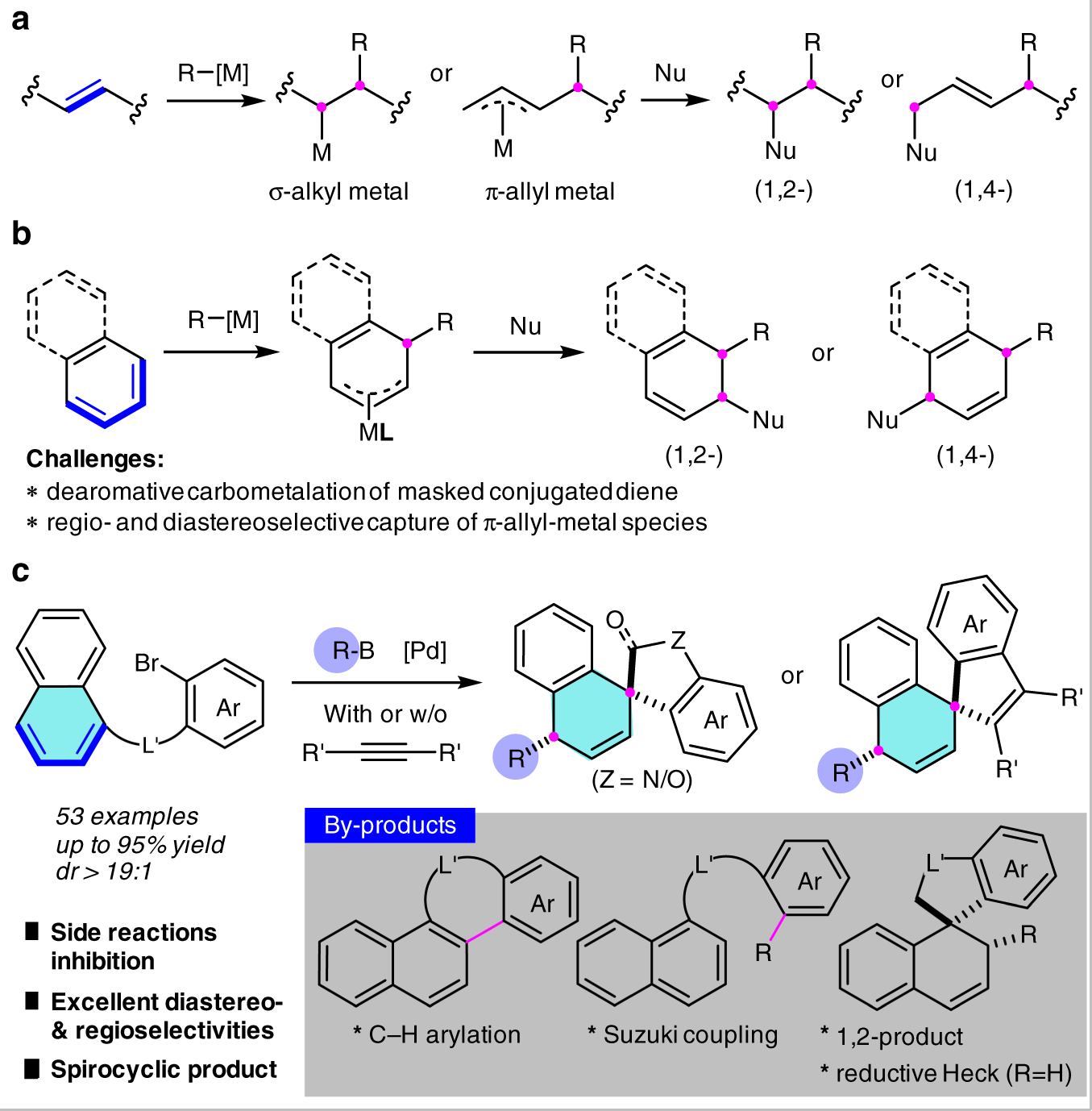
Dearomative 1,4-difunctionalization of naphthalenes via palladium-catalyzed tandem Heck/Suzuki coupling reaction | Nature Communications
![PDF] Synthesis of highly substituted pyrrolidines via palladium-catalyzed cyclization of 5-vinyloxazolidinones and activated alkenes | Semantic Scholar PDF] Synthesis of highly substituted pyrrolidines via palladium-catalyzed cyclization of 5-vinyloxazolidinones and activated alkenes | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/cc304cad8d2ab97404cd61ddbf74a80f56fbda8c/3-Figure2-1.png)
PDF] Synthesis of highly substituted pyrrolidines via palladium-catalyzed cyclization of 5-vinyloxazolidinones and activated alkenes | Semantic Scholar

Palladium-catalyzed enantioselective addition of two distinct nucleophiles across alkenes capable of quinone methide formation. | Semantic Scholar

Nickel-catalyzed allylic carbonylative coupling of alkyl zinc reagents with tert-butyl isocyanide | Nature Communications

Mechanistic approaches to palladium-catalyzed alkene difunctionalization reactions. - Abstract - Europe PMC
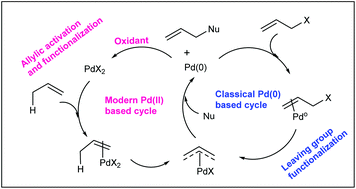
Catalytic allylic functionalization via π-allyl palladium chemistry - Organic & Biomolecular Chemistry (RSC Publishing)

Palladium‐Catalyzed Oxidation Reactions of Alkenes with Green Oxidants - Hu - 2019 - ChemSusChem - Wiley Online Library

Asymmetric allylic substitution by chiral palladium catalysts: Which is more reactive, major π-allyl Pd(II) species or minor π-allyl species? - ScienceDirect
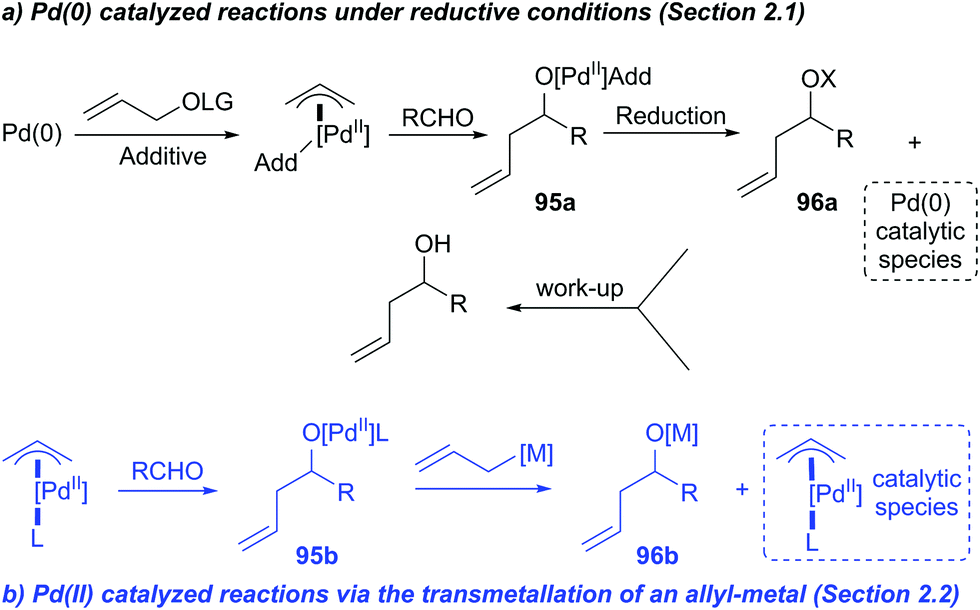
Catalytic nucleophilic 'umpoled' π-allyl reagents - Chemical Society Reviews (RSC Publishing) DOI:10.1039/C7CS00449D
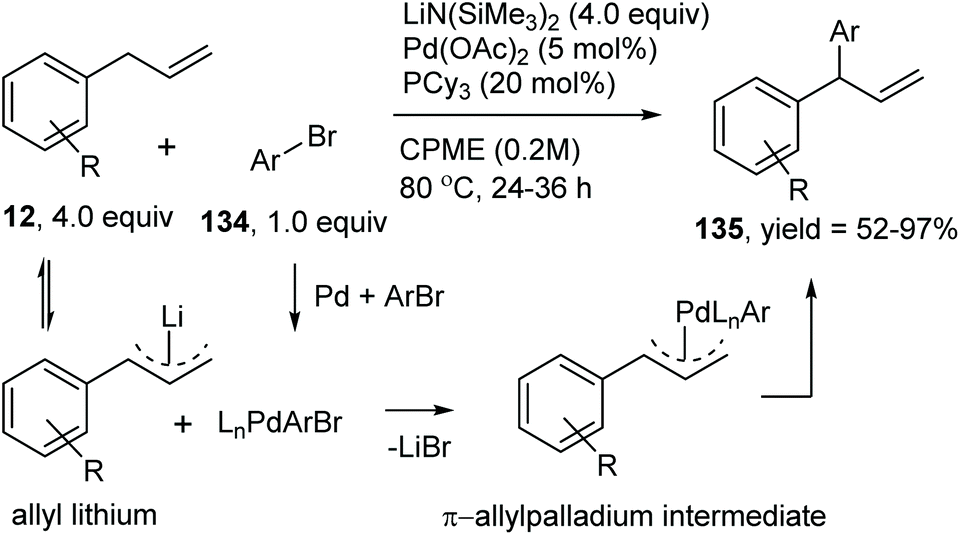
Catalytic allylic functionalization via π-allyl palladium chemistry - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C9OB01725A

Palladium-catalyzed reaction of γ-silylated allyl acetates proceeding through 1,2-shift of a substituent on silicon - ScienceDirect

Synthesis, characterization, and reactivity of (π-allyl)palladium(II) wrap-around complexes with 1,3-dienes - ScienceDirect